Intranasal Foralumab
Other Inflammatory Indications
Combination Study Of Ozempic & Nasal Anti-CD3 (Foralumab)
In October 2024 we announced positive results demonstrating the anti-inflammatory potential of our anti-CD3 antibody (foralumab) in combination with semaglutide, a GLP-1 agonist marketed by Novo Nordisk (NYSE: NVO) under the brand names Ozempic and Wegovy. The data show that the combination of nasal anti-CD3 plus semaglutide improves liver homeostasis and reduces inflammation in models of diet-induced obesity (DIO), providing a potential novel approach to combat obesity-related inflammation, and liver inflammation and dysfunction.
Key Highlights:
- Nasal anti-CD3 in combination with semaglutide demonstrates synergistic effects in promoting liver homeostasis in preclinical models of diet-induced obesity.
- The combination significantly reduces inflammation markers, a key factor in obesity-related metabolic disorders.
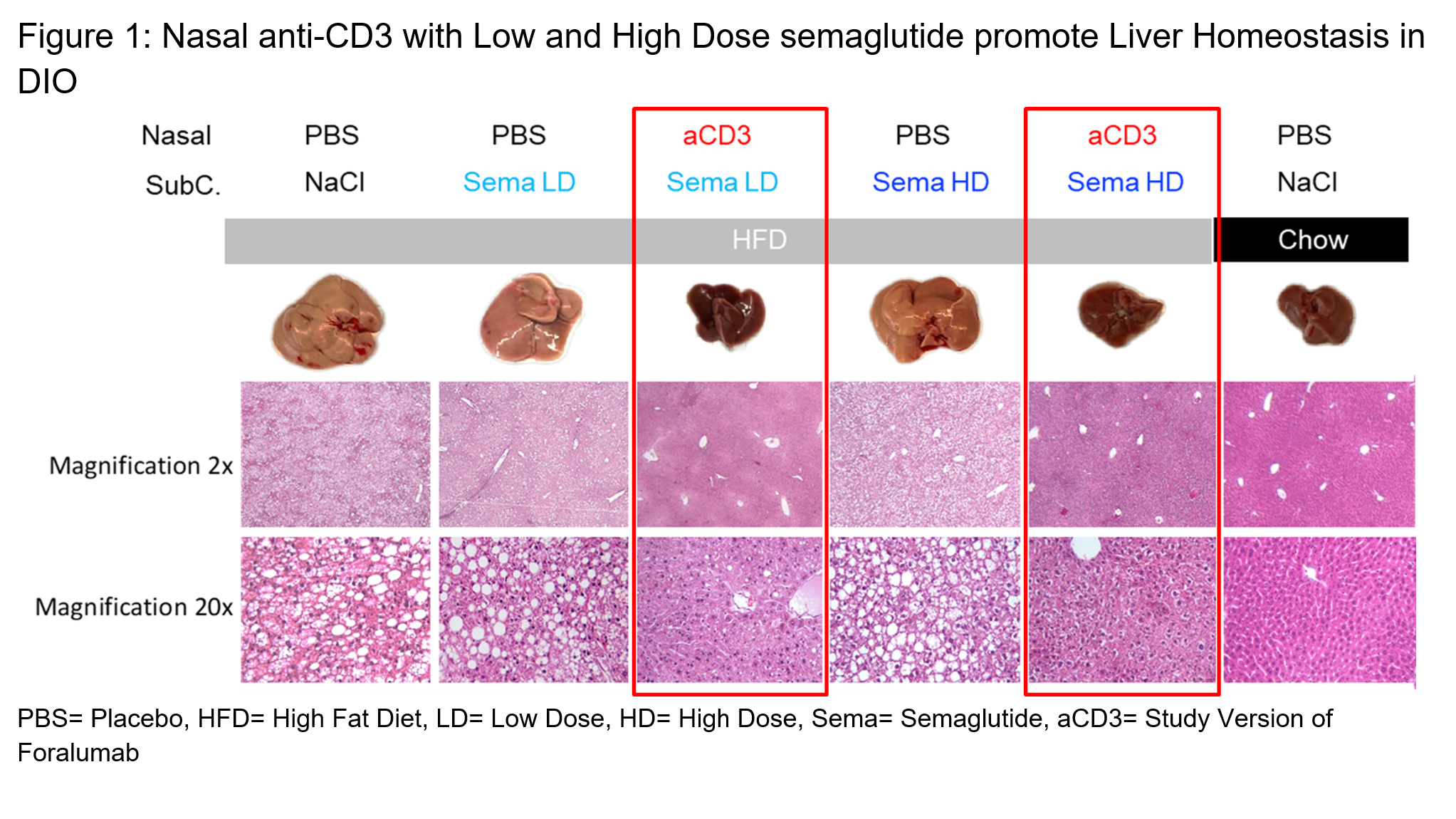
In Figure 1, the far-right column shows the explanted liver and histology of that liver at two magnifications for a mouse fed a low-fat chow “normal” diet (“lean mouse”). The dark and smaller liver on the right is a typical healthy liver from a lean mouse. All the mice under the gray bar in the figure were fed a high fat chow (“HFD”) resulting in diet induced obesity. As the columns outlined by red boxes demonstrate, administration of the combination of nasal anti-CD3 and semaglutide had livers that looked more like the liver from the lean mouse. The HFD mice given low dose or high dose semaglutide alone had enlarged fatty livers that were more similar to the HFD control.
The full study, conducted by Dr Howard Weiner and Selma Boulenouar PhD, and a research team at Brigham and Women’s Hospital, Boston, Massachusetts, demonstrates that nasal anti-CD3 in combination with Semaglutide, helps restore liver homeostasis in diet induced obesity models where liver dysfunction and inflammation are prominent. The combination therapy led to marked reductions in pro-inflammatory cytokines and significant improvements in liver markers associated with metabolic regulation. This suggests a dual benefit in both managing obesity and preventing its associated inflammation-related complications.
The complete findings from this study are being submitted to a peer-reviewed journal for publication.
Full Press Release: https://www.globenewswire.com/news-release/2024/10/30/2971693/0/en/Tiziana-Life-Sciences-Announces-Positive-Results-from-Ozempic-and-Nasal-Anti-CD3-Combination-Study.html
Covid 19
We have completed a Phase 2 trial treating mild to moderate non-hospitalized COVID-19 patients in Brazil with intranasal foralumab with positive results.
Immune hyperactivity is an important contributing factor to the morbidity and mortality of COVID-19 infection. Nasal administration of anti-CD3 monoclonal antibody downregulates hyperactive immune responses in animal models of autoimmunity through its immunomodulatory properties. We performed a randomized pilot study of fully-human nasal anti-CD3 (Foralumab) in patients with mild to moderate COVID-19 to determine if its immunomodulatory properties had ameliorating effects on disease.
Thirty-nine outpatients with mild to moderate COVID-19 were recruited at Santa Casa de Misericordia de Santos in Sao Paulo State, Brazil. Patients were randomized to three cohorts: 1) Control, no Foralumab (n=16); 2) Nasal Foralumab (100ug/day) given for 10 consecutive days with 6 mg dexamethasone given on days 1-3 (n=11); and 3) Nasal Foralumab alone (100ug/day) given for 10 consecutive days (n=12). Patients continued standard of care medication.
We observed reduction of serum IL-6 and C-reactive protein in Foralumab alone vs. untreated or Foralumab/Dexa treated patients. More rapid clearance of lung infiltrates as measured by chest CT was observed in Foralumab and Foralumab/Dexa treated subjects vs. those that did not receive Foralumab. Foralumab treatment was well-tolerated with no severe adverse events.

Traumatic Brain Injury (TBI)

Crohns disease: Novimmune Clinical Trials using Intravenous Foralumab
Intravenous Foralumab has been studied in one Phase 1 and two Phase 2 clinical trials (Crohn’s disease https://clinicaltrials.gov/ct2/show/NCT00630643 and patients With Acute Renal Allograft Rejection https://clinicaltrials.gov/ct2/show/NCT00805909) conducted by Novimmune. A total of 68 patients were administered Foralumab:
- The short-term tolerability profile of Foralumab was very similar to those reported with other anti-CD3 antibodies and no new emerging concerns have been identified.
- Total daily doses of up to 1mg (~ 500 µg/m2) per patient were generally well tolerated without corticosteroid premedication with reduction in the Crohn’s Disease Activity Index (CDAI) scores in patients.
- The most common adverse events following exposure to Foralumab were Infusion Related Reactions (IRRs).
- A clear reduction of IRRs was observed with steroid pre-medication up to 2.5 mg/dose for 5 consecutive days.
- Therefore, usage of steroid pre-medication allows the administration of higher doses.
- Both the magnitude and duration of CD3 modulation increased in a dose related manner.
- No anti-drug antibodies were detected.
Completed Phase 1 Clinical Trial: Oral Administration of Foralumab in Healthy Volunteers
An enteric-coated capsule formulation has been developed for oral administration of Foralumab. cGMP manufacturing of clinical trial materials for a Phase 1 study has been completed. The Phase 1 clinical trial for Foralumab in healthy volunteers is a single-center, single-arm, ascending dose study in which low doses (1.25, 2.5 and 5.0 mg/dose) of Foralumab and placebo were orally administered. The primary endpoint of the Phase 1 study was safety and tolerability of oral Foralumab in humans. A Phase 1 trial of the oral, enteric capsule formulation of Foralumab in healthy subjects was initiated on December 2, 2019. Results of the Phase 1 Trial were reported on January 20, 2020 The proprietary oral formulation, comprising the lyophilized and stabilized free-flowing powder of formulated Foralumab encapsulated in an enteric-coated capsule, was well-tolerated at all doses tested and there were no drug-related safety issues even at the highest dose of 5 mg in this trial.